|
First known in India 3,000 years ago, diamonds were considered to be mystical minerals ever since and they are still the "kings of precious stones".
A diamond is in general seen as the precious stone, that fetches highest prices and it concentrates the highest value on tiniest room. An example among various others is a rough red diamond of 1.75 ct (carat = 0.2 g) that was sold in 1997 in an auction by Christies in Geneva for 805,000 $. Approximately 24 tons of natural diamonds per year are produced today. This represents a value of six to seven billions of $.

Not all of them are manufactured into jewellery but the share of this branch is about 90 percent considering the sales of the world’s gemstone sector and it represents a few billions of $. The larger rest of the produced natural diamonds are used as a valuable and superhard industrial material.
The large esteem of a diamond as precious stone and industrial material derives from its unique and special physical properties. They are established by the crystal chemical (chemical and crystallographic – crystal structural) properties. That is why they are presented and explained first.
Chemically seen a diamond is pure carbon, C as chemical symbol, ordinal number 6 (it is the sixth element in the periodic system), and therefore the atom has six electrons (1s2 2s2 2p1x 2p1y = every two s-electrons in the first and second layer in pairs and two p-electrons with unpaired spin) and a relative atomic weight of 12. The diamond in comparison to graphite, that also consists of carbon, is under high pressure (dependent on temperatures around 1,200 °C with some 10,000 bar) the more stable modification. It was formed under these conditions in basic rocks (peridotite = olivine-garnet-rock and eclogite = pyroxen-garnet-rock) of the upper earth’s mantle in depth of 200 km and more. The growth of diamond crystals in the rock of the upper mantle of the earth took place already two billions of years ago under geological very old and strong layers of the crust of our planet – the cratones. In geological significant younger ages diamonds together with fragments of the surrounding rock were often blown to the surface through so called pipes by volcanic eruptions that were rich of gas. Brecciated fillings of rock, predominately kimberlites, then are the carrying layers with a diamond concentration of under 0.2 ct to sometimes 8 ct per ton of rock. The deposits of diamonds in the secondary deposition products from weather-beaten primary deposits, loose and firm sands or conglomerates as soaps, prove their robustness. A diamond is chemically resistant to acids and bases. Above 800 to 850 °C at air it burns to carbon dioxide.
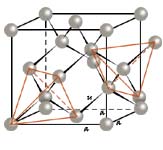
The crystal structure of a diamond can be described geometrically in a way that the carbon atoms occupy the positions of two cubic face - centred lattice that are merged one into another so that they seem to be shifted against each other a quarter of a cubic diagonal of the elementary cell. Each carbon atom of the second partial grid occupies the positions of every second tetrahedral gap of the first partial lattice. This is also the case the other way round so that all carbon atoms are coordinated tetrahedrically by four next neighbour atoms. The cubic hexakisoctahedral crystal class m3m and the space group Fd3m follow from the crystal structure.
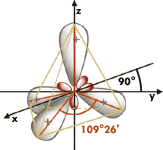 The angle of 109°28’ - tetrahedron angle (= the double of the angle between the cubic diagonal and the edge of the cube) - between the connecting lines of two neighbour atoms derives as well geometrically from the crystal structure as from the orbits of the four electrons of the carbon atom’s second layer that are sp3 – hybridised (a paired s-electron mutates to an unpaired p-electron). The angle of 109°28’ - tetrahedron angle (= the double of the angle between the cubic diagonal and the edge of the cube) - between the connecting lines of two neighbour atoms derives as well geometrically from the crystal structure as from the orbits of the four electrons of the carbon atom’s second layer that are sp3 – hybridised (a paired s-electron mutates to an unpaired p-electron).
 If you assume that the carbon atoms as a rigid sphere would even touch each other, a very low packing density of just 34% would be the result. On the other hand, a space filling of over 90% for the diamond structure together with a very strong covalent bond that explains some of the unique properties such as the extreme hardness arises from the callotte -like overlapping of the atoms’ bond orbits. From the lattice constant of a diamond, a = 0.355 nm, with eight carbon atoms per elementary cell follows a distance of 0.154 nm between the centres of two neighbour atoms. If you assume that the carbon atoms as a rigid sphere would even touch each other, a very low packing density of just 34% would be the result. On the other hand, a space filling of over 90% for the diamond structure together with a very strong covalent bond that explains some of the unique properties such as the extreme hardness arises from the callotte -like overlapping of the atoms’ bond orbits. From the lattice constant of a diamond, a = 0.355 nm, with eight carbon atoms per elementary cell follows a distance of 0.154 nm between the centres of two neighbour atoms.
Among the seven simple forms of growth that are possible in the hexakisoktahedral crystal class the octahedron {111} is the most common one for the diamond crystal. This and also the good cleavage of the crystals parallel to the areas of the octahedron (# to (111)) can be explained by the analytic examination of the bond vectors of the crystal structure. Other forms are hexahedron and dodecahedron often in combination with the octahedron. The crystal’s surface and edges are often rounded, frequently arrange lamellar or in steps as a vicinal or some kind other structured surface. The reasons for this can be found predominately in the solution processes when the physicochemical conditions during their formation changed. Typical examples are so called trigons - numerous microscopically visible triangular deepenings in the octahedron’s surfaces - and occasionally rectangular deepenings in the areas of the dodecahedrons (rectangles).
These deepenings were etch pits at exit points of dislocation lines. Their contour indicates the respective surface symmetry while their number hints to the intensity of structural defects in the crystal caused by plastic deformation. Depending on the deposit the share of so called irregular formed diamonds and sometimes polycrystalline aggregates is increased. Something special are the sheet-formed diamond crystals called macles. According to the Spinel Law they are twins (twins on (111), twin plane on parallel to an octahedron face). A reiterated parallel twin formation or stacking fault according to the law explains the sheet-formation and the triangular shape of the crystals that can be found sometimes.
Among all the physical properties the extreme hardness has to be pointed out. Until today there is no known harder material. Hardness in a physical way can be defined as resistance against local force. In practice a hard indent - according to the method the point of a pyramid or a ball - is pressed at the surface with a constant load W which will create a permanent impression in a defined area A. By convention Hardness H is H = W / A. Results for diamonds with cube areas (100): 10 t mm-2 (in comparison: corundum 2 t mm-2 , copper 40 kg mm-2). Hardness as crystal property is in principle anisotrop (hardness anisotropy), i.e. directional bound reflecting the symmetry of the crystal class. In different areas the indentation hardness and also the abrasion hardness that is more important for the processing of diamonds to gemstones differ (e.g. cube {100}, rhombedodecahedron {110} and octahedron {111} ) even according to their direction. In this way for example the abrasion hardness in direction parallel to the longitudinal axis of a rhombic dodecahedron area (parallel [110]) differs a lot from the one in vertical direction (parallel [100]). The octahedron on top of this shows differences between direction and opposite direction according to the threefold area symmetry. These differences are used effectively in cutting with diamond powder.
The specific weight of diamond is 3.52 g cm-3 and consequently clearly higher than the one of silicon (2.38 g cm-3) that has the same crystal structure. Its position in the periodic system is 14 - in the fourth group below carbon - and it is an element with more than twice the atomic weight. For the diamond this is clearly shown by the high electron density in spite of the geometrically slack coordination of four which was explained in the context of the bond conditions.
The extreme refraction of light – in average the refraction index n is 2.34 – can also be explained by this. A second optical property of the diamond as a gemstone is also of importance: the dispersion of the refraction of light. The refraction index for bigger wavelengths (e.g. red at approximately 690 nm) is smaller and for smaller wavelengths it is bigger (e.g. blue 480 nm), which already creates a spectral play of colours for small prismatic inclined areas. Refraction and dispersion give the diamond its lustre and fire. For the diamonds that are most frequently manufactured into jewellery it is the brilliance, caused by the refraction (when the light enters the stone and when it leaves it) and the reflection (reflection at the surface and multiple total reflection, that depends on the wavelength at an angle of incidence from already 25°) inside the stone. According to this the diamond cut in its proportions that offer an optimal use of light and high brilliance was developed.
To judge a gemstone diamond the four characteristic – its four "c" – are used:
- "c"arat (weight)
- "c"olour
- "c"larity
- "c"ut.
The weight can be measured in a precise and fast way, in opposite to colour, clarity and cut. Until today there are no easy to handle gauges at hand or they are very expensive. But in many countries mostly similar rules were developed that allow - on the basis of experiences and in connection with modern technology - a correct judgement and a reliable certification.
The diamond has some more extraordinary properties, that are not of importance concerning its use as jewellery – instead they are used for diagnostics – but that are applicated in high-tech:
- very good heat conduction (according to the type between 600 and 2,200 Wm-1K-1 at 20°C)
- very good resistor (isolator >1014 Ωm)
- broad optical transparency (pure diamond: between 225 nm and 2.5 µm and above 6 µm)
- semiconductor with a high energy gap, to be doped with acceptors and donators
|


